"Pharmacology of serotonin: what a clinician should know" in Gut (2004) [full text]
"The pharmacology of serotonin (5-hydroxytryptamine or 5-HT) in the gut has been the centre of intense interest and research for several decades. Although it is now recognised that 5-HT is contained in intrinsic enteric neurones (where it works as a neurotransmitter), enterochromaffin cells of the mucosa are the main source (more than 90%) of the body’s 5-HT. In the gut, 5-HT is an important mucosal signalling molecule targeting enterocytes, smooth muscle cells, and enteric neurones. Application of exogenous 5-HT evokes so many responses that it is difficult to determine which are physiologically relevant. This bewildering range of effects is largely due to the presence of multiple receptor subtypes, which appear to be present on several classes of myenteric neurones, on smooth muscle cells, and on epithelial cells. 5-HT is thought to be involved in the pathophysiology of several clinical entities such as functional gut disorders (namely, irritable bowel syndrome), carcinoid diarrhoea, and chemotherapy induced emesis. In this review, the possible targets for pharmacological intervention are analysed in the light of the most recent advances of our understanding of the role of 5-HT in gut pathophysiology. Indeed, the recent regulatory interventions on cisapride (a 5-HT4 receptor partial agonist) and alosetron (a 5-HT3 receptor antagonist) have prompted a rethinking of our approaches to the pharmacological modulation of serotonergic pathways. In gut disorders, the most interesting targets for pharmacological intervention are:(1) the 5-HT receptor subtypes known to affect gut function such as those belonging to the 5-HT1, 5-HT3, 5-HT4, and 5-HT7 subtypes; and (2) the 5-HT reuptake mechanism which, apart from the central nervous system, is expressed in enteric neurones and enterocytes and is blocked by antidepressants.
...
Concerning possible pathophysiological roles of SERT [serotonin transport], apart from changes in the expression or pharmacological profile of SERT associated with dysfunctions of central serotonergic transmission (for example, depression and migraine), it is noteworthy that in guinea pigs with experimental colitis, a concomitant increment in 5-HT availability and a decrease in mRNA SERT expression were detected in the inflamed colonic mucosa.175 Clinical evidence suggests that similar alterations may also occur in patients with either IBS or inflammatory bowel disease.176 Moreover, SERT polymorphisms may be responsible for pharmacogenetic differences, as suggested by the colonic transit response to alosetron in patients with diarrhoea predominant IBS.177" [Emphasis mine.]
Concerning possible pathophysiological roles of SERT [serotonin transport], apart from changes in the expression or pharmacological profile of SERT associated with dysfunctions of central serotonergic transmission (for example, depression and migraine), it is noteworthy that in guinea pigs with experimental colitis, a concomitant increment in 5-HT availability and a decrease in mRNA SERT expression were detected in the inflamed colonic mucosa.175 Clinical evidence suggests that similar alterations may also occur in patients with either IBS or inflammatory bowel disease.176 Moreover, SERT polymorphisms may be responsible for pharmacogenetic differences, as suggested by the colonic transit response to alosetron in patients with diarrhoea predominant IBS.177" [Emphasis mine.]
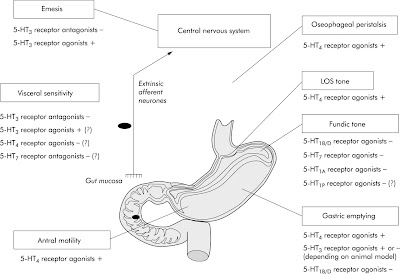
From this article "Pharmacology of serotonin," don't miss Table 1 entitled "Main serotonin (5-hydroxytryptamine or 5-HT) receptor subtypes in the gut".
"Serotonin synthesis and uptake in symptomatic patients with Crohn's disease in remission" in Clinical Gastroenterol Hepatol (2007)
Symptoms resembling irritable bowel syndrome (IBS) are reported frequently in Crohn's disease (CD) patients in remission. Studies of the mucosal content of serotonin, which is a pivotal neurotransmitter in the gut, suggest that serotonin availability is altered in IBS patients. We aimed to study the role of serotonin in the generation of IBS-like symptoms in CD patients in remission. ...
CD patients in remission who experience IBS-like symptoms have increased mucosal TpH-1 levels in the colon, suggesting that increased serotonin biosynthesis in the colon plays a role in the generation of the symptoms."
"Amino Acid Responsive Crohn’s Disease: a case study" in Clin Exp Gasteroenterol (2010)
"In the intestinal tract of Crohn’s patients there is excessive synthesis with associated increased tissue levels of serotonin.8,9. In Crohn’s disease high levels of serotonin dominate synthesis, metabolism and transport leading to dopamine and catecholamine levels that are low relative to the balance needed to function properly with the serotonin levels present. ...
There is a known genetic defect of the organic cation transporters, OCTN1 and OCTN2, in the colon of patients suffering from Crohn’s diease. All OCT and OCTN transporters are capable of transporting organic cations including serotonin, dopamine, and their precursors.8 In Crohn’s disease the serotonin content of the mucosa and submucosa of the proximal and distal colon is increased. Increased synthesis of serotonin is known to be associated with Crohn’s disease. No reasonable explanation of the etiology of serotonin elevation in the colon tissue of Crohn’s disease patients has been put forth previously.
It is postulated that the known OCTN1 and OCTN2 genetic deficit may be tied to the increased synthesis and tissue levels of serotonin seen with Crohn’s disease. Based on OCT assay interpretation, it appears that a severe imbalance between serotonin and dopamine transport, synthesis, and metabolism is at the heart of Crohn’s disease.
Imbalance of the serotonin-dopamine transport system has been linked to numerous diseases. It is purposed that much of the clinical constellation found with Crohn’s disease may be induced by a serotonin toxicity of the colon exacerbated by relatively low levels of dopamine resulting from defective OCTN transport....
There appears to be a defect in transport of serotonin precursors of the colon. Serotonin precursors are transported preferentially at the exclusion of dopamine precursors leading to high levels of synthesis, high levels of serotonin in portions of the colon, and compromise of catecholamine synthesis. Properly balancing the serotonin and dopamine precursor transport leads to less serotonin synthesis, less serotonin in the tissue of the proximal and distal colon, along with increase synthesis of dopamine, norepinephrine, and epinephrine. Increased serotonin levels of Crohn’s disease lead to increased monoamine oxidase activity (MAO) which without reciprocal increases of the catecholamines leads to increased metabolism of the catecholamines further exacerbating the imbalance.
... [T]he patient was then taking the following in divided daily doses: 300 mg 5-HTP, 6000 mg L-tyrosine, 240 mg L-dopa, and 4500 mg L-cysteine with cofactors. Following this change in amino acid dosing values, the patient continued to be asymptomatic, a state that exists to this day as long as he is compliant with the prescribed amino acid dosing values." [Emphasis mine.]
No comments:
Post a Comment